autozone survey sweepstakes
hentai meet n fuck kingdom
so you want to fuck my sister
prodigy hack bookmarklet
for more freebies visit bie.org © buck institute for education project rubric
lana pawn loan
american asian dating
fuck date usa reviews
husband watches wife fuck
the sweepstakes audit bureau
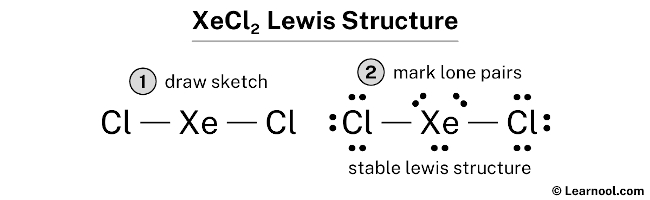
XeCl2 is the chemical formula for Xenon Dichloride. It is an inorganic compound consisting of a xenon atom bonded to two chlorine atoms. Understanding the molecular structure of XeCl2 is essential in predicting its chemical properties and reactivity. To determine the Lewis structure of XeCl2, we first need to know the total number of valence electrons. Xenon is in Group 18 of the periodic table and has 8 valence electrons. Chlorine is in Group 17 and has 7 valence electrons. Multiplying the number of chlorine atoms by their valence electrons, we get a total of 2 x 7 = 14 valence electrons. Adding this to the 8 valence electrons of xenon, we have a total of 22 valence electrons. Using this information, we can now construct the Lewis structure of XeCl2. We start by placing xenon in the center and drawing a single bond to each chlorine atom. This uses up 4 valence electrons, leaving us with 18 remaining. Next, we place 3 lone pairs of electrons around each chlorine atom, accounting for 12 more electrons. Finally, we distribute the remaining 6 electrons as lone pairs around the xenon atom. The resulting Lewis structure for XeCl2 is as follows: Xe | Cl- Xe - Cl | In this structure, the xenon atom is surrounded by two chlorine atoms and has three lone pairs of electrons. The chlorine atoms each have three lone pairs of electrons as well. This arrangement allows for a stable electron configuration and minimizes electron repulsion. Now, lets analyze the molecular geometry of XeCl2. The shape of a molecule is determined by the arrangement of its atoms and lone pairs of electrons. In the case of XeCl2, we have a central atom (xenon) bonded to two other atoms (chlorine). The presence of lone pairs of electrons around the central atom further influences the shape. The molecular geometry of XeCl2 can be determined using the VSEPR theory (Valence Shell Electron Pair Repulsion theory). According to VSEPR, electron pairs (whether bonding or lone pairs) repel each other and tend to be as far away from each other as possible. This repulsion leads to specific molecular shapes. In XeCl2, the xenon atom has three lone pairs of electrons. These lone pairs exert a greater repulsion force on the bonding electron pairs, causing the bond angles to deviate from the ideal 109.5 degrees (typical for tetrahedral geometry). The repulsion from the lone pairs pushes the bonding pairs closer together, resulting in a bent molecular geometry. The molecular geometry of XeCl2 is therefore bent or V-shaped. The bond angle between the two chlorine atoms is less than 109.5 degrees due to the repulsion from the lone pairs of electrons. This bent shape is a result of the electron pair repulsion theory, which seeks to minimize electron-electron repulsion within the molecule. Understanding the molecular geometry of XeCl2 is crucial in predicting its physical and chemical properties. The bent shape of XeCl2 affects its polarity and reactivity. Since the chlorine atoms are more electronegative than xenon, they pull the shared electrons towards themselves, creating a dipole moment. As a result, XeCl2 is a polar molecule, with a slightly positive charge on xenon and slightly negative charges on the chlorine atoms. This polarity affects the intermolecular forces between XeCl2 molecules, leading to specific physical properties such as boiling and melting points. Additionally, the bent molecular geometry of XeCl2 influences its reactivity, as it determines how other molecules or ions can approach and interact with the XeCl2 molecule. In conclusion, the XeCl2 molecule has a bent or V-shaped molecular geometry. This is a result of the repulsion between the lone pairs of electrons on the xenon atom and the bonding pairs of electrons between xenon and chlorine. Understanding the molecular geometry of XeCl2 is crucial in predicting its physical and chemical properties, as well as its reactivity with other molecules or ions.
A step-by-step explanation of how to draw the XeCl2 Lewis Dot Structure .. In the Lewis structure of XeCl2 structure there are a total of 22 valence electrons. XeCl2 is also called Xenon dichloride. Note that XeCl2 can have an Expanded Octet and have more than eight . xecl2 lewis structure molecular geometry. Xenon Dichloride, XeCl2 Molecular Geometry & Polarity - Tutor-Homework.com. First draw the Lewis dot structure: Electron geometry: trigonal bipyramidal xecl2 lewis structure molecular geometry. Hybridization: sp 3 d Next determine the 3D molecular geometry using VSEPR rules: Decision: The molecular geometry of XeCl 2 is linear with symmetric electron region distribution. Therefore this molecule is nonpolar.. Xecl2 Lewis Structure& Characteristics: 13 Complete Facts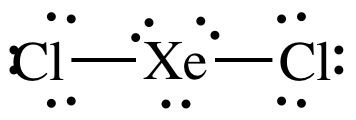
autozone survey sweepstakes
. The information on this page is fact-checked. XeCl 2 (xenon dichloride) has one xenon atom and two chlorine atoms xecl2 lewis structure molecular geometry. In the XeCl 2 Lewis structure, there are two single bonds around the xenon atom, with two chlorine atoms attached to it, and each atom has three lone pairs.. XeCl4 Lewis Structure, Geometry, Hybridization, and Polarity. It has a molecular mass of 207.2836 g mol -1hentai meet n fuck kingdom
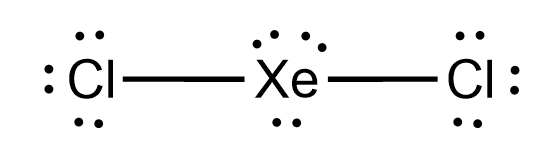
so you want to fuck my sister
. Lewis Symbolsprodigy hack bookmarklet
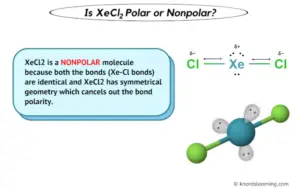
. Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. xecl2 lewis structure molecular geometry. XeCl2 Lewis Structure in 5 Steps (With Images) - Pediabay. Steps of drawing XeCl2 lewis structure Step 1: Find the total valence electrons in XeCl2 . xecl2 lewis structure molecular geometry. Is XeCl2 Polar or Nonpolar? (And Why?) xecl2 lewis structure molecular geometry. XeCl2 is a NONPOLAR molecule because both the bonds (Xe-Cl bonds) are identical and XeCl2 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of XeCl2 lewis structure and its 3D geometry.. Answered: Draw the Lewis structure of XeCl2 and… . Science Chemistry Draw the Lewis structure of XeCl2 and then determine the hybridization of the central atomfor more freebies visit bie.org © buck institute for education project rubric
. Draw the Lewis structure of XeCl2 and then determine the hybridization of the central atom. BUY Chemistry: Principles and Practice 3rd Edition ISBN: 9780534420123 Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer xecl2 lewis structure molecular geometry. SeCl2 Lewis Structure, Molecular Geometry, Bond Angle, Polar or .. It discusses the molecular geometry and bond angle for the SeCl2 lewis structure. The video also mentions the molecular geometry and bond angle for SeCl2. This video shows you how to draw the lewis structure for SeCl2 - Selenium Dichloride.. Lewis Structure of XeCl2 - Root Memory. The lewis structure of XeCl 2 contains two single bonds, with xenon in the center, and two chlorines on either side. There are three lone pairs on both the chlorine atom and xenon atom.lana pawn loan
. Is XeCl2 Polar or Nonpolar? - Science Coverage xecl2 lewis structure molecular geometry. Molecular geometry. The central atom of XeCl2 (Xe) has a total of 10 electrons localized in the form of 4 as Xe-Cl bonding and 6 as non-bonding pairs. And due to the symmetrical structure, the polarity of the Xe-Cl bond cancel each other and no dipole charge induced on the molecule.. Lewis Dot Structure for XeOF2 (and Molecular Geometry) xecl2 lewis structure molecular geometry. A step-by-step explanation of how to draw the XeOF2 Lewis Dot Structure (Xenon oxydifluoride).For the XeOF2 structure use the periodic table to find the tota.. What is the Lewis structure for XeCl2? . Best Answer. Copy. Xenon dichloride - XeCl2 : LewisGeometryAnalysis. The geometry of XeCl2 is linear with a symmetric charge distributionamerican asian dating
. Therefore this molecule is nonpolar xecl2 lewis structure molecular geometry. Wiki User. ∙ 2012 .. BeCl2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and .. BeCl2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. BeCl2 referred to as Beryllium Chloride, is an inorganic compound. It appears as white or yellow crystal solid at room temperature xecl2 lewis structure molecular geometry. It can exist in both monomeric and 1-D polymeric forms.. Solved valence electrons, lewis structure, electron | Chegg.com. valence electrons, lewis structure, electron geometry, molecular geometry, bond angles, polar or non polar XeF4 H2O I3^- SO2 AsF5 IF3 XeCl2 This problem has been solved! Youll get a detailed solution from a subject matter expert that helps you learn core concepts. xecl2 lewis structure molecular geometry. Solved For the following compounds: XeCl2 and SCl4 Give . Chemistry questions and answers. For the following compounds: XeCl2 and SCl4 Give the: a) the Lewis structure b) The electron group geometry c) The molecular geometry d) A 3-D representation e) annotate the 3D representation with bond dipoles and molecular dipoles.fuck date usa reviews
. Solved 5. Refer to your Lewis structure for SeCl2 What is | Chegg.com xecl2 lewis structure molecular geometry. 5. Refer to your Lewis structure for SeCl2 What is the "molecular geometry" for selenium dichloride? bent linear tetrahedral square planar trigonal planar.. Solved Consider the following molecule. Draw the Lewis - Chegg. Electronic configuration of Xe = [Kr] 4d105s25p6 Where [Kr] is the elctronic configuration of Krypton. Valence electron is the electron in the last shell of an element = last shell electron = 2+6 = …. Consider the following molecule. Draw the Lewis Structure and use that to fill in the following chart: XeCl2 Number of Valence e (Whole .. What is the value of the bond angle in XeCl2?. Question: What is the value of the bond angle in XeCl2? Answer and Explanation: Become a Study.com member to unlock this answer! Create your account View this answer 180° is the value of the bond.. Solved Draw the Lewis structure for each of the following - Chegg. rules for making shapes of compounds 1)central element is least electrone …. Draw the Lewis structure for each of the following and then determine the shape (molecular geometry) of the molecule or ion indicated. Do not include formal charges in your drawing. XeF4 : shape = ChemDoodle ChemDoodle -] -000- rx-. SeH2 : shape =. xecl2 lewis structure molecular geometry. Do all of the following molecules contain at least one bond angle at .. SeS3 The Lewis structure of SeS3 is (from www.homeworklib.com) This is an AX3 molecule, and its geometry is trigonal planar. All bond angles in a trigonal planar molecule are approximately 120 °. SeS2 The Lewis structure of SeS2 is :. . S = xecl2 lewis structure molecular geometry. . Se = xecl2 lewis structure molecular geometry. . S: This is an AX2E molecule. Its electron geometry is trigonal planar.. XeCl4 Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair .. By Madhusudan DN In this post, well go through how to build the xecl4 lewis structure, formal charge, hybridization, and geometry step by step. The chemical formula for xenon tetrachloride is XeCl4 xecl2 lewis structure molecular geometry. The elements xenon and chlorine belong to the noble gas and halogen family groupings, respectively, in the periodic table. xecl2 lewis structure molecular geometry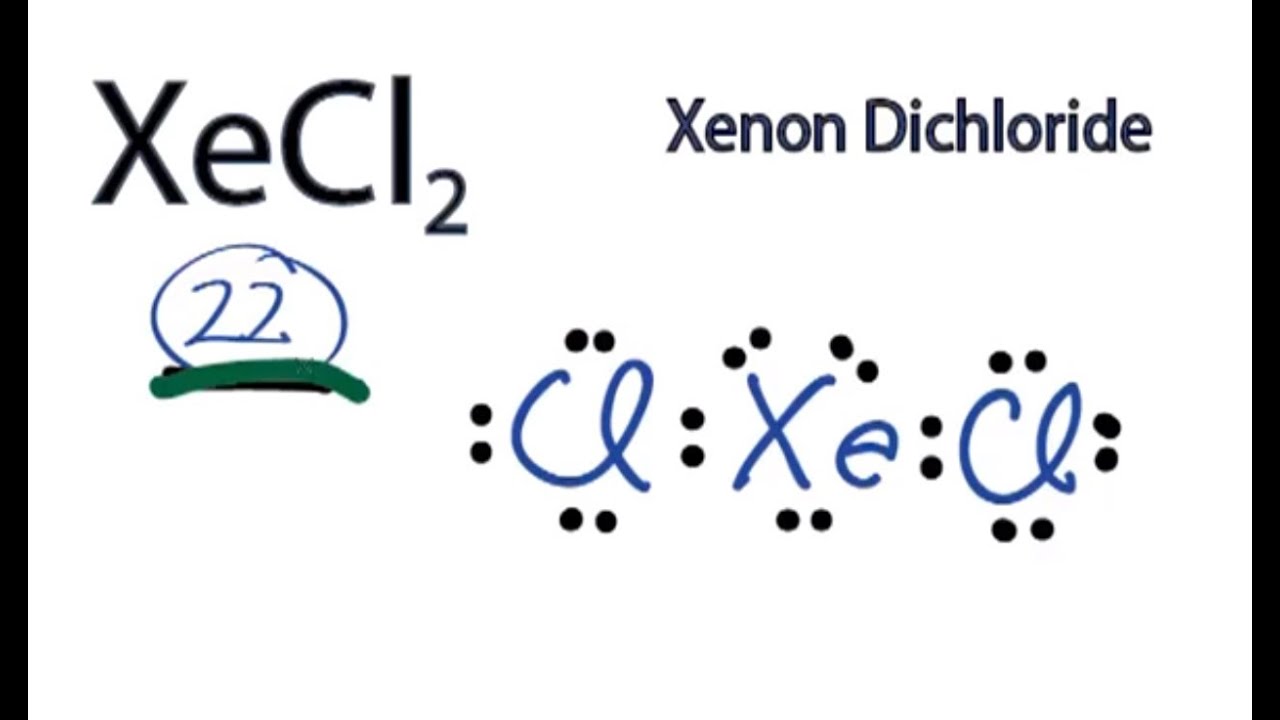
husband watches wife fuck
. XeF2 Lewis Structure, Molecular Geometry . - Techiescientist xecl2 lewis structure molecular geometry. XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram xecl2 lewis structure molecular geometry. XeF2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. This is an active solvent and is found to be soluble in different fluorides like HF and bromine pentafluoride. If we look at the process of synthesis of xenon difluoride, heres .. Find the number of electron pairs, number of lone pairs, electronic .
the sweepstakes audit bureau
. 7. b. 9. c. 5. d xecl2 lewis structure molecular geometry. 6. e. 8. Find the number of valance electrons and draw the Lewis dot structure for BeCl_2. Given the following Lewis .. 10, 11 chem test review Flashcards | Quizlet xecl2 lewis structure molecular geometry. Use the bond energies provided to estimate ΔH°rxn for the reaction below. CH3OH (l) + 2 O2 (g) → CO2 (g) + 2 H2O (g)ΔH°rxn = ? -392 kJ. A double covalent bond contains. 2 pairs of electrons xecl2 lewis structure molecular geometry. Identify the number of bonding pairs and lone pairs of electrons in O2. 2 bonding pairs and 4 lone pairs. Place the following elements in order of .. Water Molecular Geometry and Bond Angles - YouTube. Lewis Structures, Introduction, Formal Charge, Molecular Geometry, Resonance, Polar or Nonpolar The Organic Chemistry Tutor 806K views 6 years ago VSEPR Theory - Basic Introduction The. xecl2 lewis structure molecular geometry. SO4 2- Molecular Geometry / Shape and Bond Angles - YouTube